Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kano, Koichi*; Hagiwara, Satoshi*; Igarashi, Takahiro; Otani, Minoru*
Electrochimica Acta, 377, p.138121_1 - 138121_10, 2021/05
Times Cited Count:17 Percentile:71.29(Electrochemistry)We investigated the free corrosion potential at an interface between an Al electrode and an aqueous NaCl solution under acidic conditions via density functional theory combined with the effective screening medium and reference interaction site model (ESM-RISM). The electrode potentials for the anodic and cathodic corrosion reactions were obtained from the grand potential profile as a function of the electron chemical potential at the interface. Thereafter, we determined the free corrosion potential using the Tafel extrapolation method. The results of the free corrosion potential were consistent with previous experimental data. By controlling the pH, we determined the pH dependence of the free corrosion potential, and the results agreed well with the experimental results. Our results indicated that the ESM-RISM method duly described the environmental effect of an acidic solution and precisely determined the free corrosion potential.
Sakamoto, Tomokazu*; Matsumura, Daiju; Asazawa, Koichiro*; Martinez, U.*; Serov, A.*; Artyushkova, K.*; Atanassov, P.*; Tamura, Kazuhisa; Nishihata, Yasuo; Tanaka, Hirohisa*
Electrochimica Acta, 163, p.116 - 122, 2015/05
Times Cited Count:57 Percentile:82.8(Electrochemistry)Kitatsuji, Yoshihiro; Otobe, Haruyoshi; Kimura, Takaumi; Kihara, Sorin*
Electrochimica Acta, 141, p.6 - 12, 2014/09
Times Cited Count:5 Percentile:9.72(Electrochemistry)Reduction processes of U(VI) in weakly acidic solutions were investigated based on electrochemical and spectrophotometric measurements. A reversible one-electron reduction of U(VI) to U(V) and a further irreversible reduction of U(V) were observed voltammetrically at a gold microdisk electrode in solutions of pH from 2.0 to 3.5. Aggregates of U(IV) were formed as a deposit on the electrode and a colloid in the bulk solution, when the electrolysis was carried out at a gold gauze electrode, even though the potential applied was that available for the first one-electron reduction wave of U(VI) observed. It was elucidated that the aggregate was produced by the combination of the one-electron reduction to U(V) and the disproportionation of U(V) producing U(IV) and U(VI). The aggregate enhanced the rate of the disproportionation of U(V), and hence the reduction current of U(VI) increased abruptly when a definite amount of aggregate was formed on the electrode, in the solution, or both.
Chen, R.*; Tanaka, Hisashi*; Kawamoto, Toru*; Asai, Miyuki*; Fukushima, Chikako*; Na, H.*; Kurihara, Masato*; Watanabe, Masayuki; Arisaka, Makoto; Nankawa, Takuya
Electrochimica Acta, 87, p.119 - 125, 2013/01
Times Cited Count:108 Percentile:94.92(Electrochemistry)A novel electrochemical adsorption system using a nanoparticle film of copper (II) hexacyanoferrate (III) was proposed for selectively removing cesium from wastewater. This system can be used for cesium separation without extra chemical reagents or any filtration treatment. Cesium uptake and elution can be simply controlled by switching the applied potentials between anodes and cathodes. Data from batch kinetic studies well fitted the intraparticle diffusion equation, reflecting a two-step process: a steepest ascent portion followed by a plateau extending to the equilibrium. The effective cesium removal with a high distribution coefficient (

 5
5 10
10 mL/g) can be adopted in a large pH range from 0.3 to 9.2, and in the presence of several diverse coexisting alkaline cations, suggesting it can be taken as a promising technology for actual nuclear wastewater treatment.
mL/g) can be adopted in a large pH range from 0.3 to 9.2, and in the presence of several diverse coexisting alkaline cations, suggesting it can be taken as a promising technology for actual nuclear wastewater treatment.
Kiuchi, Hisao*; Niwa, Hideharu*; Kobayashi, Masaki*; Harada, Yoshihisa*; Oshima, Masaharu*; Chokai, Masayuki*; Nabae, Yuta*; Kuroki, Shigeki*; Kakimoto, Masaaki*; Ikeda, Takashi; et al.
Electrochimica Acta, 82(1), p.291 - 295, 2012/10
Times Cited Count:14 Percentile:34.42(Electrochemistry)We study the characteristics of oxygen adsorption on metal-free carbon-based cathode catalysts derived from nitrogen-containing polyamide (PA) and nitrogen-free phenolic resin (PhRs). Electrochemical analysis and Raman spectroscopy showed higher 2-electron oxygen reduction reaction (ORR) activity and more defect sites in PA than PhRs. The increase in the amount of adsorbed oxygen in PA was also identified by oxygen adsorption isotherms.  X-ray photoelectron spectroscopy reveals that graphite-like nitrogen contributes to oxygen adsorption and C=O components are dominant in PA. These experimental results indicate that the adsorbed C=O components near the graphite-like nitrogen can be assigned as active sites for 2-electron ORR.
X-ray photoelectron spectroscopy reveals that graphite-like nitrogen contributes to oxygen adsorption and C=O components are dominant in PA. These experimental results indicate that the adsorbed C=O components near the graphite-like nitrogen can be assigned as active sites for 2-electron ORR.
Kitatsuji, Yoshihiro; Kimura, Takaumi; Kihara, Sorin*
Electrochimica Acta, 74, p.215 - 221, 2012/07
Times Cited Count:2 Percentile:5.39(Electrochemistry)Redox reactions of U, Np and Pu ions of various oxidation states were investigated by flow electrolysis at a column electrode (CE) equipped with a platinized glassy carbon (GC) fiber working electrode (Pt/GC-WE), and compared with those observed at the CE with an ordinary activated GC fiber working electrode (GC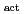 -WE). Since the overpotential for the reduction of NpO
-WE). Since the overpotential for the reduction of NpO
 and PuO
and PuO
 were decreased when Pt/GC-WE was employed, the one-electron reduction of NpO
were decreased when Pt/GC-WE was employed, the one-electron reduction of NpO
 to Np
to Np followed by that of Np
followed by that of Np to Np
to Np and the one-step three-electron reduction of PuO
and the one-step three-electron reduction of PuO
 to Pu
to Pu proceeded at the CE with Pt/GC-WE, different from the reduction processes of NpO
proceeded at the CE with Pt/GC-WE, different from the reduction processes of NpO
 and PuO
and PuO
 at the CE with GC
at the CE with GC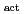 -WE. A rapid and precise method for the preparation of U, Np and Pu ion of a desired oxidation state was proposed by taking advantage of the unique characteristics of the CE with Pt/GC-WE.
-WE. A rapid and precise method for the preparation of U, Np and Pu ion of a desired oxidation state was proposed by taking advantage of the unique characteristics of the CE with Pt/GC-WE.
Kobayashi, Masaki*; Niwa, Hideharu*; Saito, Makoto*; Harada, Yoshihisa*; Oshima, Masaharu*; Ofuchi, Hironori*; Terakura, Kiyoyuki*; Ikeda, Takashi; Koshigoe, Yuka*; Ozaki, Junichi*; et al.
Electrochimica Acta, 74, p.254 - 259, 2012/07
Times Cited Count:52 Percentile:80.98(Electrochemistry)The electronic structure of the residual metal atoms in FePc-based carbon catalysts, prepared by pyrolyzing a mixture of FePc and phenolic resin polymer at 800 C, before and after acid washing have been investigated using XAFS spectroscopy to clarify the role of Fe in the ORR activity. The decomposition analyses for the XAFS spectra reveal that the composition ratio of each Fe component is unaltered by the acid washing, indicating that the residual Fe components were removed by the acid washing irrespective of their chemical states. Because the oxygen reduction potential was approximately unchanged by the acid washing, the residual Fe itself does not seem to contribute directly to the ORR activity. The residual Fe can act as a catalyst to accelerate the growth of the sp
C, before and after acid washing have been investigated using XAFS spectroscopy to clarify the role of Fe in the ORR activity. The decomposition analyses for the XAFS spectra reveal that the composition ratio of each Fe component is unaltered by the acid washing, indicating that the residual Fe components were removed by the acid washing irrespective of their chemical states. Because the oxygen reduction potential was approximately unchanged by the acid washing, the residual Fe itself does not seem to contribute directly to the ORR activity. The residual Fe can act as a catalyst to accelerate the growth of the sp carbon network during pyrolysis. The results imply that light elements are components of the ORR active sites in the FePc-based carbon catalysts.
carbon network during pyrolysis. The results imply that light elements are components of the ORR active sites in the FePc-based carbon catalysts.
 X-ray reflectometry and LiNi
X-ray reflectometry and LiNi Co
Co O
O epitaxial film electrode synthesized by pulsed laser deposition method
epitaxial film electrode synthesized by pulsed laser deposition methodHirayama, Masaaki*; Sakamoto, Kazuyuki*; Hiraide, Tetsuya*; Mori, Daisuke*; Yamada, Atsuo*; Kanno, Ryoji*; Sonoyama, Noriyuki*; Tamura, Kazuhisa; Mizuki, Junichiro
Electrochimica Acta, 53(2), p.871 - 881, 2007/12
Times Cited Count:44 Percentile:67.69(Electrochemistry)An  experimental technique was developed for detecting structure changes at the electrode/electrolyte interface of lithium cell using synchrotron X-ray reflectometry and two-dimensional model electrodes with a restricted lattice plane. The electrode was constructed with an epitaxial film of LiNi
experimental technique was developed for detecting structure changes at the electrode/electrolyte interface of lithium cell using synchrotron X-ray reflectometry and two-dimensional model electrodes with a restricted lattice plane. The electrode was constructed with an epitaxial film of LiNi Co
Co O
O synthesized by the pulsed laser deposition method. These films provided an ideal reaction field suitable for detecting structure changes at the electrode/electrolyte interface during the electrochemical reaction. The X-ray reflectometry indicated a formation of a thin-film layer at the LiNi
synthesized by the pulsed laser deposition method. These films provided an ideal reaction field suitable for detecting structure changes at the electrode/electrolyte interface during the electrochemical reaction. The X-ray reflectometry indicated a formation of a thin-film layer at the LiNi Co
Co O
O (1 1 0)/electrolyte interface during the first charge-discharge cycle, while the LiNi
(1 1 0)/electrolyte interface during the first charge-discharge cycle, while the LiNi Co
Co O
O (0 0 3) surface showed an increase in the surface roughness without forming the surface thin-film layer.
(0 0 3) surface showed an increase in the surface roughness without forming the surface thin-film layer.

Tamura, Kazuhisa; Oko, Yoshihisa*; Kawamura, Hiroyuki; Yoshikawa, Hideki*; Tatsuma, Tetsu*; Fujishima, Akira*; Mizuki, Junichiro
Electrochimica Acta, 52(24), p.6938 - 6942, 2007/08
Times Cited Count:26 Percentile:48.64(Electrochemistry)The electrochemical behaviour of TiO under X-ray irradiation was studied. Under X-ray irradiation a current, a negative shift in the rest potential, and electrochemical oxidation and decomposition of [Fe
under X-ray irradiation was studied. Under X-ray irradiation a current, a negative shift in the rest potential, and electrochemical oxidation and decomposition of [Fe (CN)
(CN) ]
] were clearly observed. The incident photon-current conversion efficiency and energy conversion efficiency was 400-2000% and 0.2-2%, respectively, depending on sample conditions. These results show that the photoelectrochemical reactions were promoted by X-rays with a high incident photon-current conversion efficiency. The photocurrent and photopotential were observed above 4.965 keV, which corresponds to the Ti-K edge, indicating that electron-hole pairs are formed during the relaxation process of the excited Ti atoms.
were clearly observed. The incident photon-current conversion efficiency and energy conversion efficiency was 400-2000% and 0.2-2%, respectively, depending on sample conditions. These results show that the photoelectrochemical reactions were promoted by X-rays with a high incident photon-current conversion efficiency. The photocurrent and photopotential were observed above 4.965 keV, which corresponds to the Ti-K edge, indicating that electron-hole pairs are formed during the relaxation process of the excited Ti atoms.
Kuznetsov, S. A.*; Hayashi, Hirokazu; Minato, Kazuo; Gaune-Escard, M.*
Electrochimica Acta, 51(13), p.2463 - 2470, 2006/03
Times Cited Count:90 Percentile:85.75(Electrochemistry)Electrochemical experiments were carried out at 723-823K in order to estimate separation coefficients in LiCl-KCl eutectic melt containing uranium and lanthanum trichlorides. The diffusion coefficients of U(III) were determined by electrochemical methods. The standard rate constants of charge transfer for electroreduction of uranium U(III) +3e =U were calculated by the impedance spectroscopy method. It was shown that for the calculation of uranium and lanthanum separation coefficients it is necessary to determine the voltammetric peak potentials of U(III) and La(III), their concentration in the melt and the kinetic parameters relating to U(III) discharge such as transfer and diffusion coefficients, and standard rate constants of charge transfer.
=U were calculated by the impedance spectroscopy method. It was shown that for the calculation of uranium and lanthanum separation coefficients it is necessary to determine the voltammetric peak potentials of U(III) and La(III), their concentration in the melt and the kinetic parameters relating to U(III) discharge such as transfer and diffusion coefficients, and standard rate constants of charge transfer.
Kondo, Toshihiro*; Tamura, Kazuhisa*; Takahashi, Masamitsu; Mizuki, Junichiro; Uosaki, Kohei*
Electrochimica Acta, 47(19), p.3075 - 3080, 2002/07
Times Cited Count:30 Percentile:56.86(Electrochemistry)A nwely designed spectroelectrochemical cell was constructed for surface X-ray scattering (SXS) to study single crystal electrodes. Electrochemical characteristics of a specific face of a single crystal electrode can be investigated in the meniscus mode, adn SXS measurement can be easily carried out using the spectroelectrochemical cell. The usefulness of the present cell was demonstrated by studying the electrochemical deposition of Pd thin layers on Au(111) and Au(001) that require precise amounts of Pd deposits.
Shibutani, Hideo*; Higashijima, Takahiro*; Ezaki, H.*; Morinaga, Masahiko*; Kikuchi, Keiichi
Electrochimica Acta, 43(21), p.3235 - 3239, 2002/00
None
 CuO
CuO by flow-coulometry
by flow-coulometrySasaki, Yuji
Electrochimica Acta, 42(12), p.1915 - 1920, 1997/00
Times Cited Count:2 Percentile:12.18(Electrochemistry)no abstracts in English